The use of terminology changes, from prescribing equipment to recommending equipment had a purposeful foundation in empowering participants of NDIS to have choice and control. This does not mean that the primary therapist is not responsible for the prescription of the potential solution or that the role of determining the clinical features falls to the supplier. As the therapist engaged to recommend solutions, we need to ensure that the end user has choice and control but also that they can make informed decisions based on our ability to complete and articulate though sound clinical reasoning and being able to critically look at potential equipment solutions to base recommendation on.
Who is the prescriber?
To answer this question, we first need to explore what is ment by the term prescription within the assessment and supply process. Lets consider a standard definition of prescription.
Prescription [prɪˈskrɪpʃ(ə)n]
NOUN
-
- an instruction written by a medical practitioner that authorizes a patient to be issued with a medicine or treatment.
(google definition)
Yes very medical sounding, so it isn’t surprizing that we can associate the implementation of this terminology from a medical funding model.
In the USA the funding system for AT continues to be based on a medical necessity model. The process requires the Primary Care Physician (often a GP) to identify the need for and type of mobility base depending on the injury or diagnosis. The Physician works with a seating and mobility specialist therapist to complete the initial prescription of a code that relates to the type of wheelchair. ( E.Michael, T.Sytsma and R.Cowan 2020 ) identifed that ensuring funding and optimal outcomes requires collaboration with an experienced seating specialist. Just like in Australasia the process “includes extensive documentation of the patient’s abilities and health needs and rationale for each part of the wheelchair selected.” (Michael et al 2020) however as our main funding is based on a functional model, more consideration is put into the functional capacity of the user.
So why do we call it a prescription if it doesn’t originate from a medical practitioner and is not a medicine or treatment but an Assistive Technology support. Are we wrong to continue this language? Again this is where terminology can be confusing. Although equipment in Australia and New Zealand is funded under a functional model it is important to also acknowledge that wheelchairs are regulated Medical devices. The Australian regulatory body is called the Therapeutic Goods Administration or (TGA) when a medical device is registered it is given a Australian Registered therapeutic Good number or ARTG number. TGA defines a medical device as;
“Section 41BD What is a medical device
(1) A medical device is:
(a) any instrument, apparatus, appliance, software, implant, reagent, material or other article (whether used alone or in combination, and including the software necessary for its proper application) intended, by the person under whose name it is or is to be supplied, to be used for human beings for the purpose of one or more of the following:
-
- (i) diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease;
- (ii) diagnosis, monitoring, treatment, alleviation of or compensation for an injury or disability; …..”Update to medical device definitions and requirements for system or procedure packs | Therapeutic Goods Administration (TGA)
Medsafe the New Zealand regulartory body, define a medical device in similar way ;
“Therapeutic Purpose
A product is regulated as a medical device or a medicine if the manufacturer or sponsor claims or implies a therapeutic purpose for it.
Legal Definition of Therapeutic Purpose
The legal definition of therapeutic purpose is contained in Section 4 of the Medicines Act 1981 and states:
Therapeutic purpose - means any of the following purposes, or a purpose in connection with any of the following purposes:
-
- preventing, diagnosing, monitoring, alleviating, treating, curing, or compensating for, a disease, ailment, defect, or injury; or
- influencing, inhibiting, or modifying a physiological process;
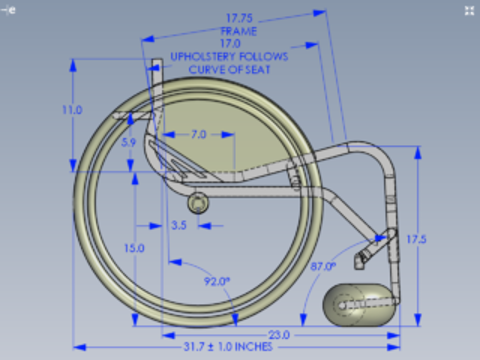 The prescription of a complex mobility base essentially refers to the specific features and accessories required to support the user’s functional outcomes, think of it like a recipe of what ingredients are required to ensure it turns out as expected. Whilst we do generally work within a functional model, the AT recommendations are still for medical devices. The form completed to ensure these features are included is most commonly referred to as the “Script”.
The prescription of a complex mobility base essentially refers to the specific features and accessories required to support the user’s functional outcomes, think of it like a recipe of what ingredients are required to ensure it turns out as expected. Whilst we do generally work within a functional model, the AT recommendations are still for medical devices. The form completed to ensure these features are included is most commonly referred to as the “Script”.
Whilst the supplier will have knowledge on the different configuration requirements and specifications of the recommended equipment, as the primary therapist engaged to make recommendations it is crucial that you are ensuring that the specific specifications, features and configurations will meet the funding criteria and support the end user goals and function. From completing a client centred assessment, you need to be able to identify and discuss different features with the end user and support them to make informed choices. When we make recommendations, we should be considering goals, lifestyle, environment, function the equipment features and users’ needs and wants.
From your initial assessment you should have a clear picture not only of the end users postural and physiological requirements but also the way the device will be operated and function. The recommending OT is still the “prescriber” or the identifier of the specific features from a clinical perspective, but it is imperative that the end user is an active participant in the process and that the equipment is not just recommended for them but that they have played an active role in the process from goal setting through to what AT they will apply to fund from their plan. To support end users to make informed choices we need to understand their functional needs and mobility devices. Solutions need to take into consideration any potential risks as poorly matched solutions not appropriatly prescribed can result harm to the end user, the outcome should be a support that increases function and meets users goals.
 If you want to understand more about the clinical determination and recommendations that guide equipment selection, make sure you are well informed and continue to develop your skills (Yes after almost 20 years I still keep learning!)
If you want to understand more about the clinical determination and recommendations that guide equipment selection, make sure you are well informed and continue to develop your skills (Yes after almost 20 years I still keep learning!)
We have a range of resources from face to face courses to on demand learning. Latest research to tools to assist you can all be found in our Clinical Services area of the Permobil website. You can also reach out to our team at any time with specific queries! (Link to clinical services pages of website)
References
- A Primary Care Provider’s Guide to Wheelchair Prescription for Persons With Spinal Cord Injury (nih.gov)
- Update to medical device definitions and requirements for system or procedure packs | Therapeutic Goods Administration (TGA)
- Medical Device Definitions (medsafe.govt.nz)
